Abstract
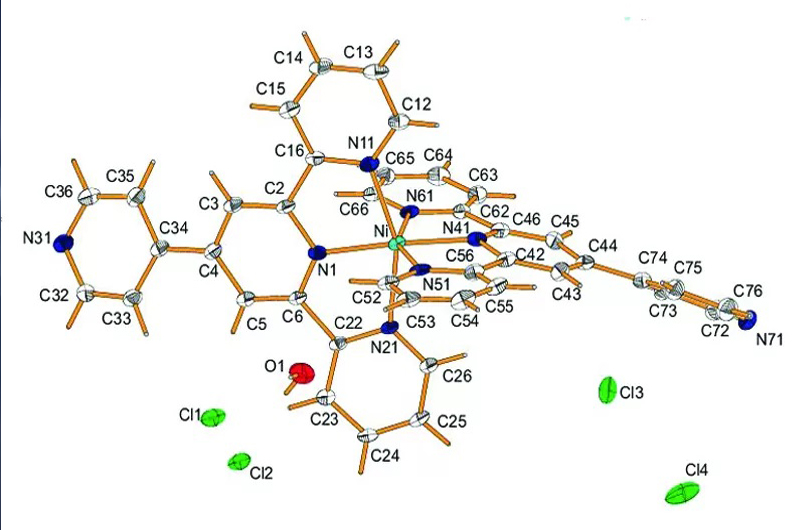
Three new d8 transitions metal complexes containing substituted-2,2′:6′,2′′-terpyridine ligands of [NiII(pytpy)2]Cl2. 4H2O (pytpy = 4′- (4-pyridyl)-2,2′:6′,2′′-terpyridine) (1), [Pt(tpyOH)Cl]+Cl–. 2H2O (2) (tpyOH = 4′-hydroxy-2,2′:6′,2′′-terpyridine) and [Pt(tpySH)Cl]+Cl–.2H2O (3) (tpySH = 4′-mercapto-2,2′:6′,2′′-terpyridine) have been prepared. The crystal structure of 1 reveals that the nickel(II) is six-coordinated by six nitrogen atoms of pytpy in a distorted octahedral geometry NiN6, while the platinum complex (2) is four-coordinated by one Cl– and three nitrogen atoms of tpyOH in a distorted square planar geometry PtClN3. The lattice crystal water molecule plays a significant structure directing role in the complexes of 1 and 2. Many strong noncovalent interactions are present in the crystal structure of 1 and 2. For example, the supramolecular network of C-H…Cl, O-H…Cl, Cl…Cl and π-π interactions connected molecules and ions in the crystalline 1, while there are several Pt…Pt, C-H…Cl, H2O…H2O, C-OH…H and π-π interactions in 2. The solution luminescence properties of 2 and 3 have been investigated. The emissions of the platinum complexes 2–3 exhibit the high-energy intense π→π* intraligand and low-energy MLCT transitions in solution. The solid-state emissions of complexes 1-3 due to the MLCT and π-π interactions are also observed in the solid state. The thermal stability of all complexes reveals that the loss of terpyridine ligand is observed at higher temperatures due to the strong metal-nitrogen bonds of terpyridine ligands.
https://www.sciencedirect.com/science/article/abs/pii/S0022286017311341